Quick Facts
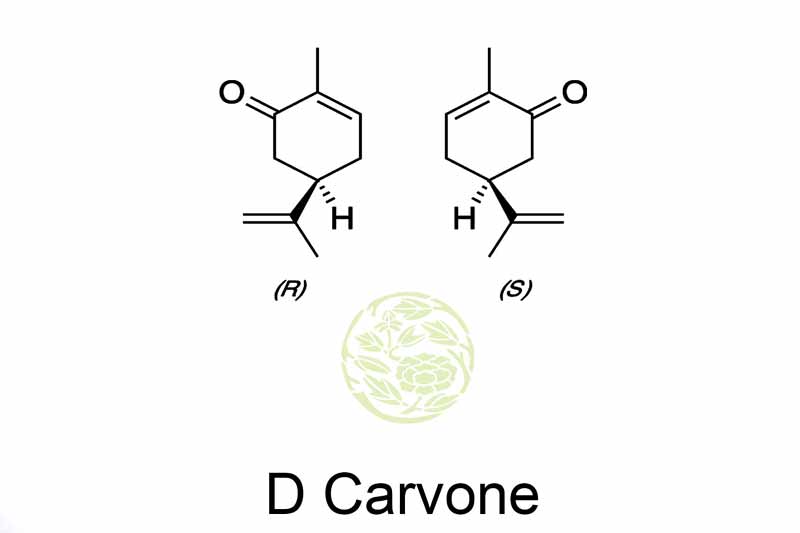
Name: D Carvone
Chemical Name: 2-Cyclohexen-1-One
Chemical Formula: C10H14O
Appearance: colourless to pale yellow liquid
Molecular Weight: __________________
Odour: Characteristic of spearmint
Specific Gravity: 0.955 to 0.960 at 25OC
GLC Purity: 99%
Refractive Index: 1.490 to 1.497 at 20OC
Colour: colourless/pale yellow
Carvone is part of the family of chemicals called terpenoids and is generally found in many essential oils. It is found in abundance in oils extracted from the seeds of caraway, spearmint and dill. There are two forms of carvone, L-carvone which smells like the spearmint leaves and D-carvone which smells like caraway seeds. D-carvone is the main constituent of the oil from caraway seeds, about 60 to 70%. The production of D-carvone is about 10 tons per year. Its presence is there in dill seed oil also (40-60%) and in mandarin orange peel oil. Some forms of oils, like the ginger grass oil contain both the enantiomers. The other natural oils which contain traces of carvones are the peppermint oil.
In use from Ancient Times
Caraway has been used for medicinal purposes by the ancient Romans but carvone was then not isolated from the caraway. The isolation of carvone happened during the time of Franz Varrentrapp in the year 1849. It was originally called carvol initially and later on Goldschmidt and Zurrer identified it as a ketone related to limonene. The structure of carvone was finally clarified by Gerog Wagner in 1894. D-carvone is prepared usually by fractional distillation of caraway oil. Even though it is different in odour and flavour, it is also distilled from dill seed oil and dill weed oil. D-Carvone is used in the production of many types of food since it has the flavour of caraway, dill and spearmint. Many of the chewing gum manufacturers use this for their flavours. Bigger producers of chewing gum, like Wrigley's, use it for Spearmint Gum etc.
Highly Toxic by Inhalation
Caraway seed is extracted with alcohol to make the European drink Kummel. D-carvone is used to prevent premature sprouting of potatoes during storage. Carvone is available cheap in both the forms because of which it is starting material for asymmetric total synthesis of natural products. It is used in synthesis of terpenoid quassin. It may cause some irritation when exposed to this compound. This compound is highly toxic by inhalation, ingestion or skin absorption. When heated to decomposition, it emits acrid smoke and toxic fumes of carbon monoxide and carbon dioxide. D-carvone blends well with fixed oils, mineral oils, propylene glycol, coriander, basil, chamomile, frankincense, ginger, lavender and orange.