Quick Facts
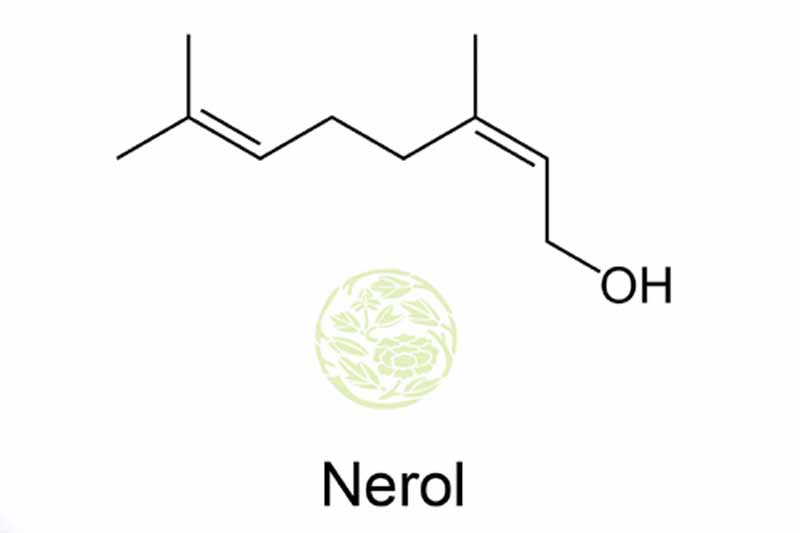
Product Name: Nerol
Chemical Name: Nerol
Chemical Formula: C10H18O
Appearance: Liquid
Refractive Index: 1.4740 to 1.4780 AT 200C
Mol. weight: 154.20
Odor: Refreshing wet sea alike
Specific Gravity: 0.8770 €“ 0.8850 AT 250C
GLC Purity:78%
Color: Colorless
Nerol is a chemical with molecular formula of C10H18O and molecular weight of 154.25grams, classified as monoterpenes with the presence of hydroxyl group €“OH at the end of the carbon chain. Nerol and Geraniol are the same substance and it shares the same molecular formula and molecular weight. These are often termed as the isomeric forms for each other. Even though they are the similar they are not 100% the same. Nerol is a cis-isomer whereas geraniol is a trans-isomer. Both of these isomers have different smell. Nerol is considered as an aroma chemical and was found in neroli essential oil. The name nerol itself was derived from this. Nerol is a colourless liquid and is often easily used in the manufacturing of perfumes.
No Characteristics Smell of essential oil
Even though nerol is present in neroli essential oil it does not have the characteristic smell of the essential oil. The aroma of nerol is more towards the smell of fresh sweet roses. Nerol is a gentle chemical and is not toxic and will not cause any kind of irritation or skin sensitizing. It is quite often used as a food flavour. A good percentage of perfumes use neroli oil as one of their main ingredients in the manufacturing of their perfumes. Some studies also say that it is use as an ingredient in Coca-Cola even though there are not confirmed reports on this. Neroli oil and nerol which is present in the oil have aroma therapeutic properties and is ideal for easing tension and anxiety.
Fresher Odor
Nerol can also be found in many other essential oils like the lemongrass oil and hops. When compared with geraniol the odour of nerol is considered to be much fresher. The isomeric with nerol is geraniol but the double bond is trans. Nerol loses water almost immediately to form dipentene. Nerol is created by pyrolysis of beta-pinene which affords mycrene. By hydrochlorinating mycrene a series of isomeric chlorides can be obtained and one of which converts to neryl acetate. Neroli oil blends well with citrus oil and many of the various floral absolutes. It also blends with many of the synthetic components available in the market.